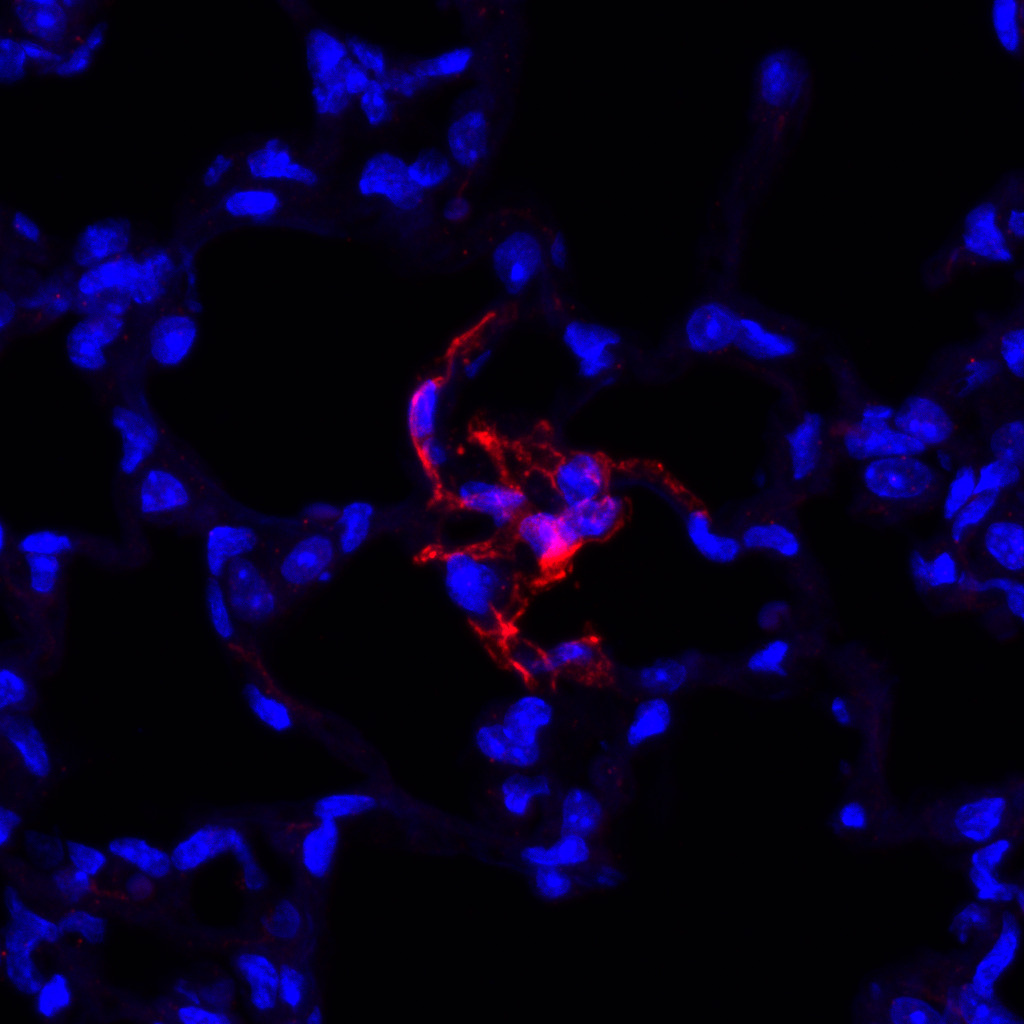
Distinct endothelial cell populations contribute differently to lung regeneration
Although endothelial cells are half of the gas exchange interface in the lung alveolus, it was previously unknown whether all endothelial cells behave similarly, or whether different populations have different functions during regeneration. Are some of them specialized to act as endothelial progenitors and regenerate the vasculature? Do some of them act as signaling hubs? Do any endothelial cells preferentially interact with the alveolar epithelium? To answer these questions, we used single-cell RNA sequencing and confocal microscopy and found that the pulmonary capillary vasculature is composed of two different cell types, a general capillary cell (CAP1) that we now know is the main endothelial progenitor after injury, and a more specialized cell (CAP2) with a larger, more complex morphology. Other groups have also identified these cells in other contexts in both mouse and human lungs. Our work and others’ have revolutionized our understanding of vascular heterogeneity in the lung and have identified many new avenues of research in lung development and regeneration.
Read the full paper on eLife
Read the full paper on eLife
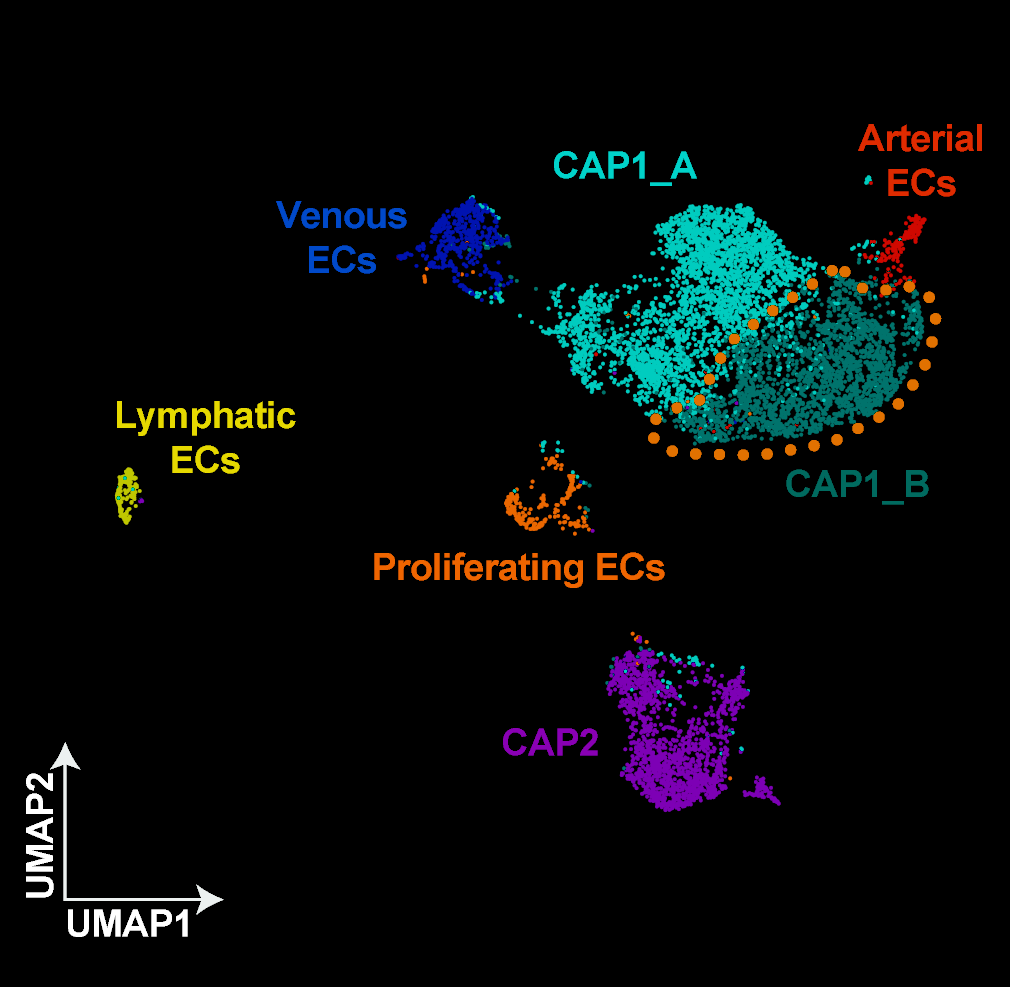
ATF3 defines a population of endothelial cells essential for lung regeneration
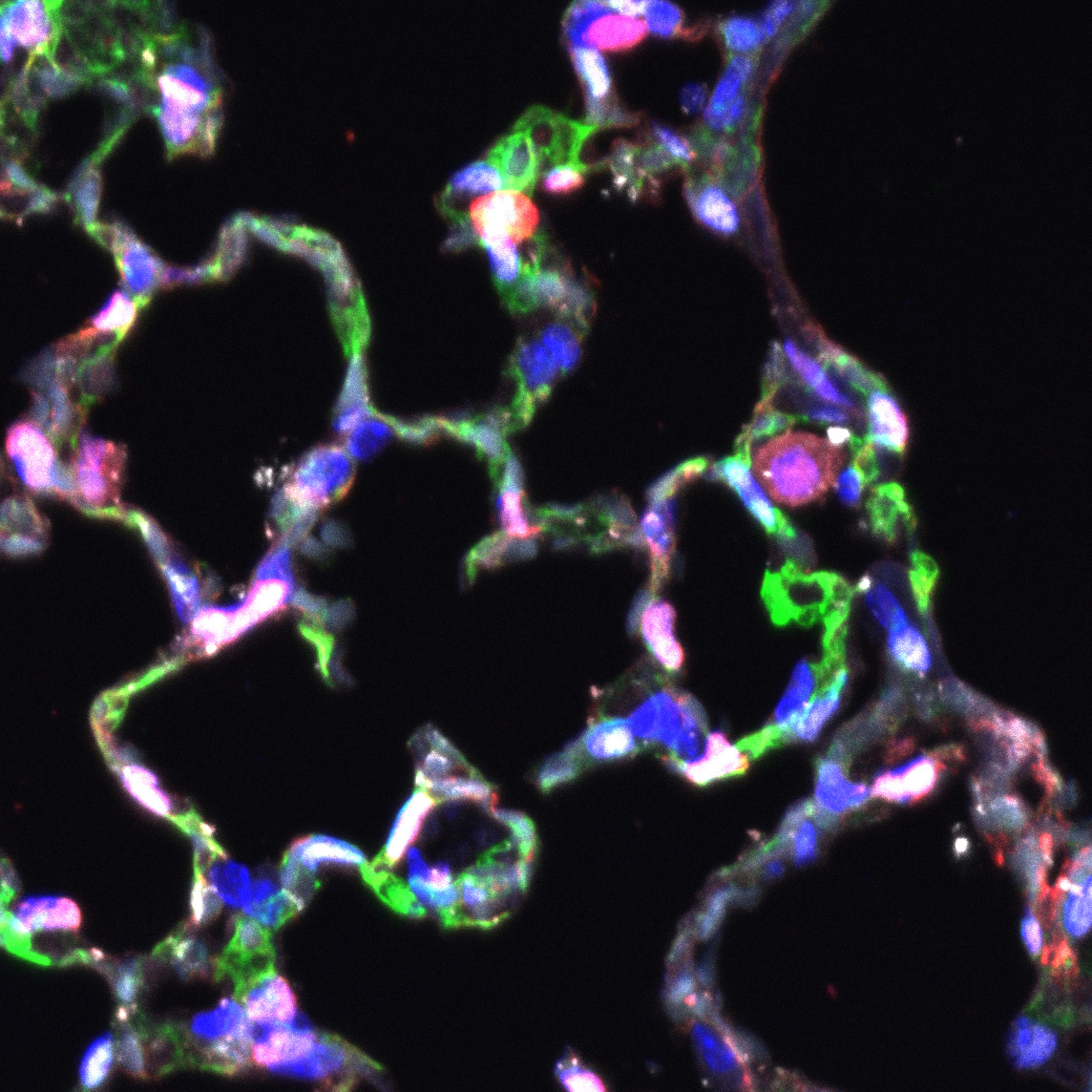
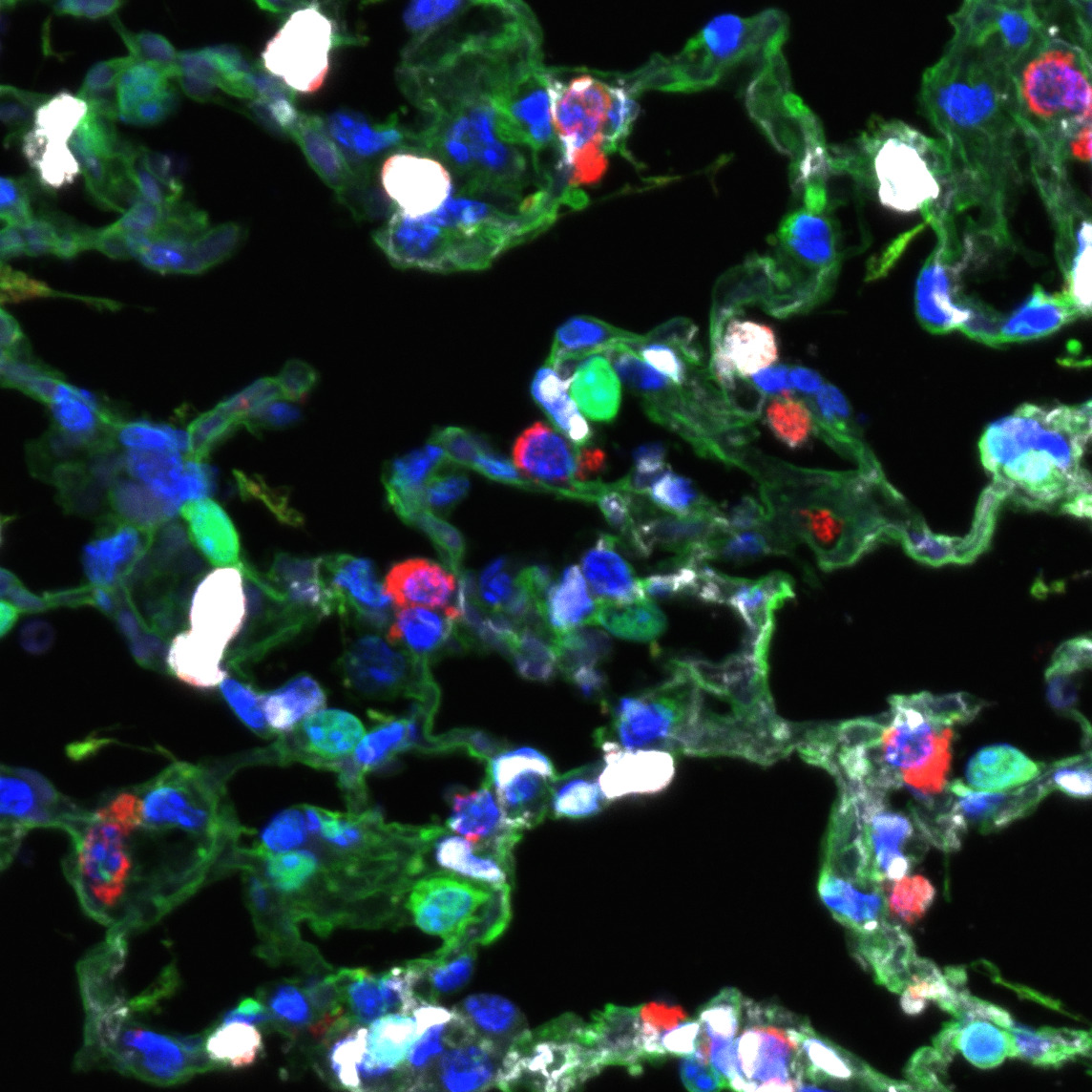
CAP1 and CAP2 endothelial cells differ in their morphology and progenitor function, and I wanted to know more about the genes and molecules driving this difference. I performed differential gene expression analysis of CAP1s and CAP2s during regeneration after influenza infection and identified a subset of CAP1s expressing high levels of the transcription factor ATF3. This CAP1 subset also expresses high levels of genes involved in angiogenesis, vascular development, and cellular stress response. ATF3-expressing endothelial cells increase in number after influenza and contribute to endothelial proliferation. When ATF3 expression is lost in the endothelium, the lung cannot regenerate. Endothelial cell signaling is altered, proliferation decreases, apoptosis increases, and an overall loss of endothelium leads to structural defects and an emphysema-like phenotype. This work identifies an essential role for the transcription factor ATF3 in lung regeneration and demonstrates that repair of the capillary endothelial network is key to restoring the structural integrity of the lung alveolus.
Read the full paper on bioRxiv
Read the full paper on bioRxiv
Endothelial cell signaling in development and regeneration of the lung
Future work in my laboratory will investigate the role of CAP2 endothelial cells during lung regeneration and identify endothelial signaling mechanisms that contribute to repair over the course of the regenerative response to influenza infection in the lung. Stay tuned for more information on these exciting projects!